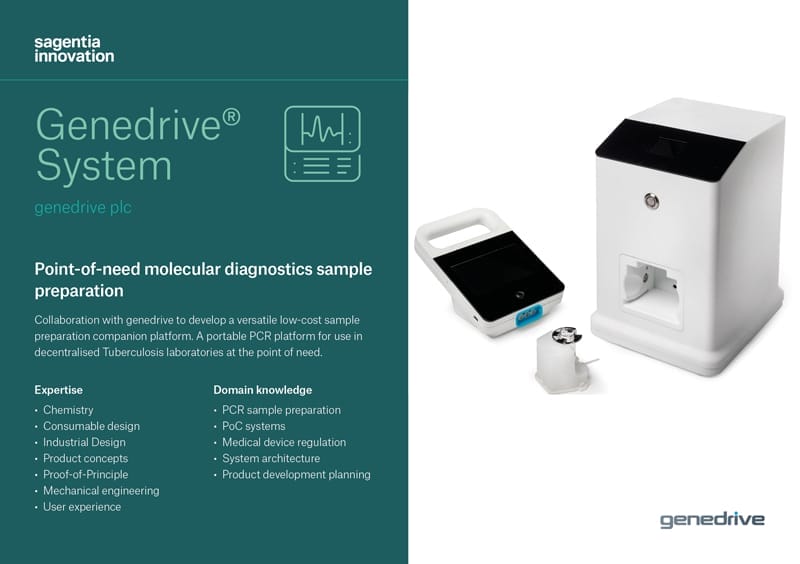
Genedrive® System
Point-of-need molecular diagnostics sample preparation
genedrive is developing its portable PCR platform for use in decentralised Tuberculosis laboratories at the point of need. Sagentia is helping to develop an associated sample processing system and secured an Innovate UK grant with genedrive
Requirement
Tuberculosis (TB) remains the most prolific global infectious disease with the greatest cause of deaths to a curable infectious agent. In 2018, the WHO reported 1.5 million deaths and in 2020, 10 million people infected. Only a small portion of global cases receive the necessary life-saving medicine.
genedrive required a low-cost, low complexity molecular tuberculosis (mTB) diagnostic system for use in decentralized settings.
The sample preparation system, alongside the Genedrive® platform, would be used to diagnose TB infection and provide drug resistance information to clinicians in a single test cartridge.
The cost of both the cartridge and instrument would need to be low enough to be affordable in the intended lower income markets.
The sample preparation system would need to process a raw sputum sample ready for diagnosis in approximately 30 minutes.
The system would need to be biosafe, handle a range of working volumes and suitable for applications additional to mTB.
Our markets

Our consultants, scientists and engineers redefine what’s possible and help R&D groups across the medical, industrial, consumer and food and beverage sectors achieve commercial return from their opportunities.
Our projects

We have completed over 10,000 projects for start-ups and global market leaders alike, from understanding the market & technology landscape through to developing and delivering complex products.

